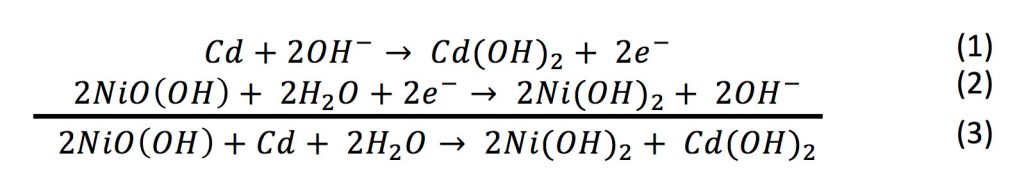Nickle cadmium battery was invented by swedish inventor waldemar jungner in 1899 and popularized and widely manufactured only during 1940 s and 1950 s.
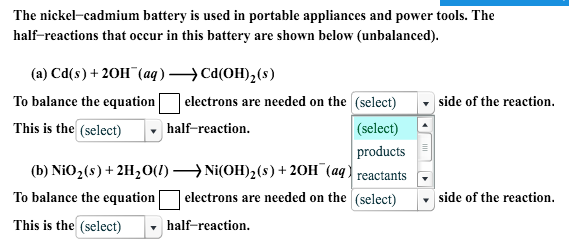
Nickel cadmium battery equation.
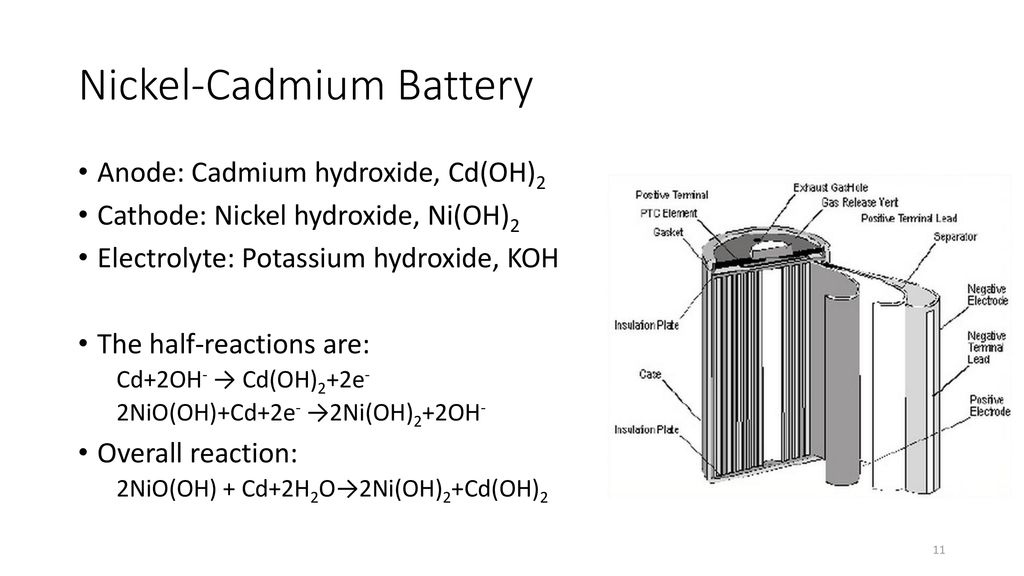
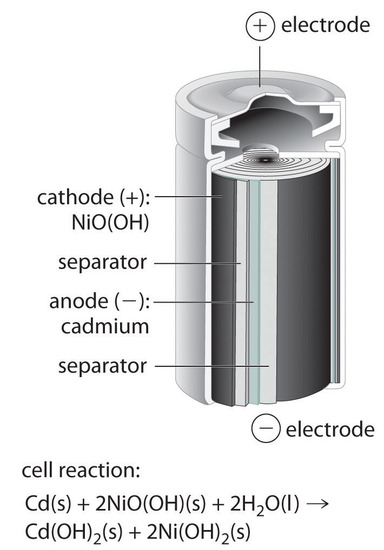
Nickle cadmium battery is a rechargeable battery with nickle oxide hydroxide and cadmium as electrodes and alkaline potassium hydroxide as electrolyte.
The maximum cell voltage during charge is 1 3 v and the average cell voltage is 1 2 v.
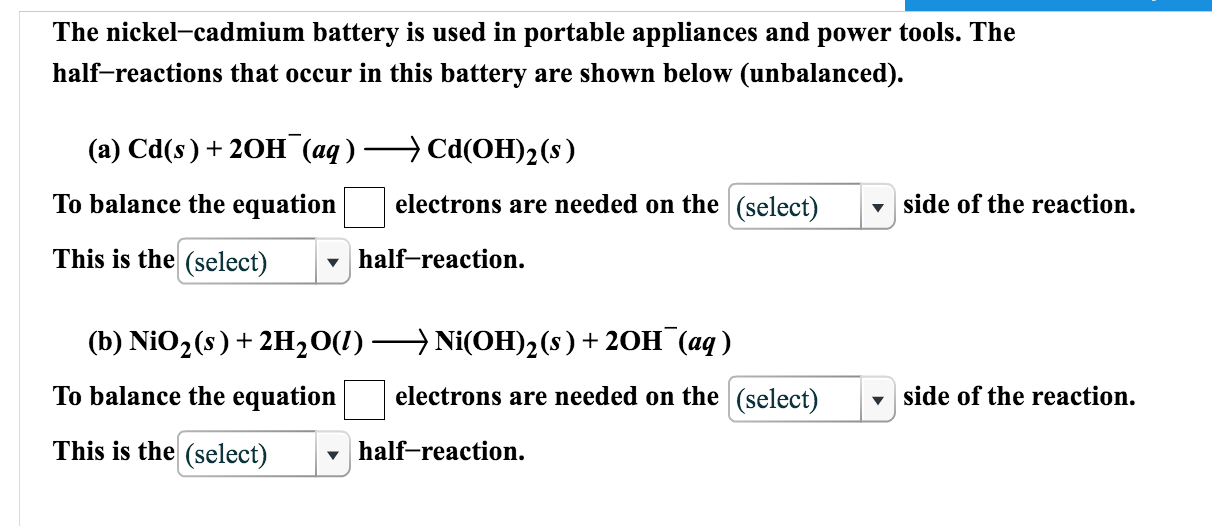
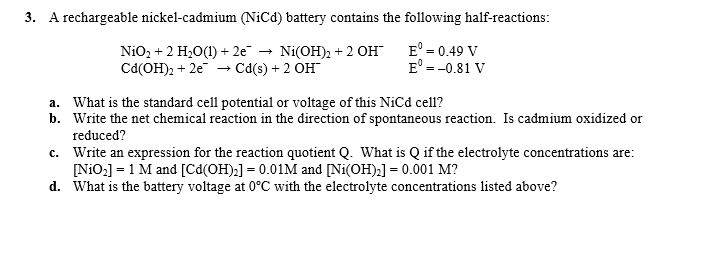
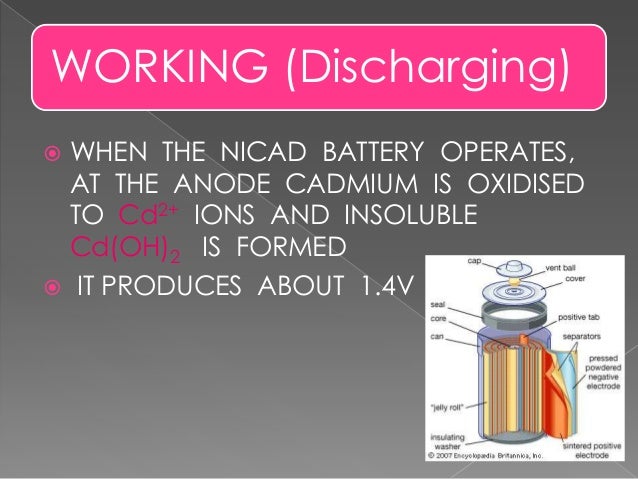
The nickel cadmium battery ni cd battery or nicad battery is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes.
Nickel cadmium batteries at the 1 2h rate yield twice the energy density of lead acid batteries i e.
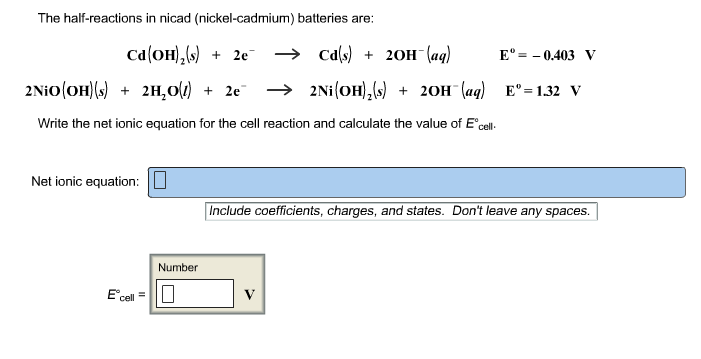
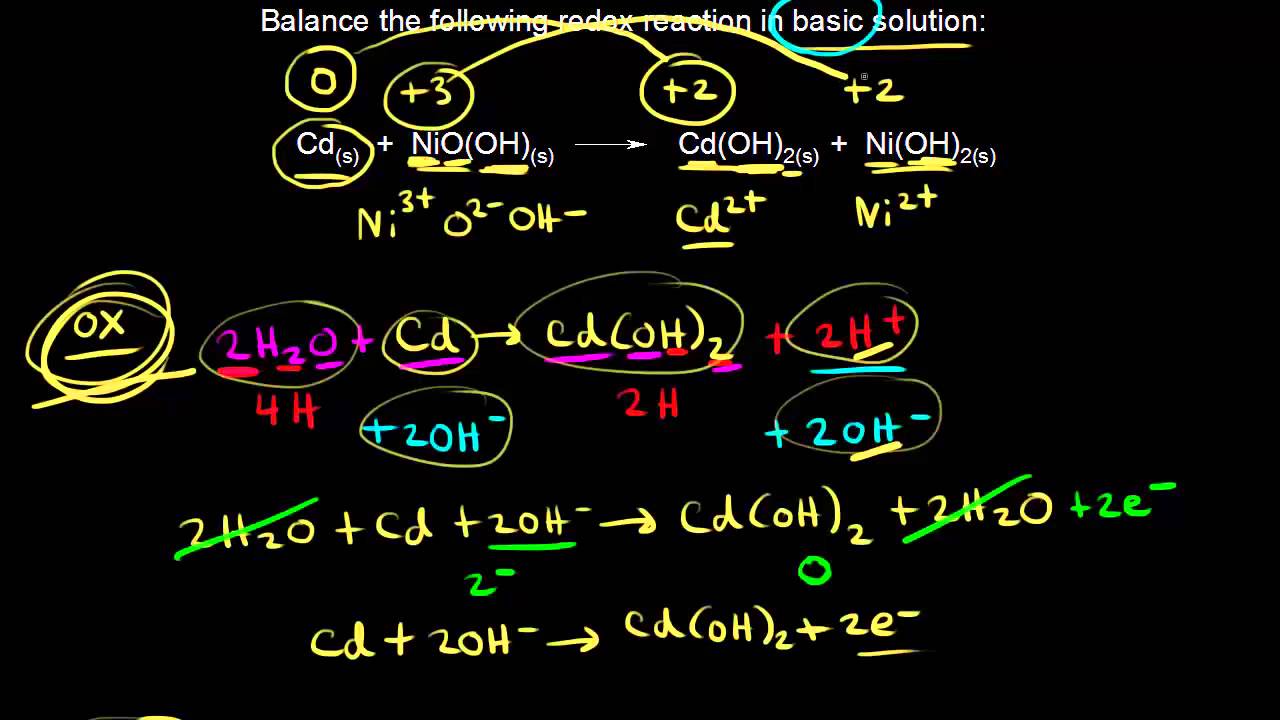
2015 ap chemistry free response 1d.
Nickel cadmium batteries are used in various developmental electric vehicles.
Battery amp hour.
Nickel cadmium battery redox reactions and electrochemistry chemistry khan academy.
So the nickel cadmium battery is like the lead storage battery it s rechargeable and therefore it can be very useful.
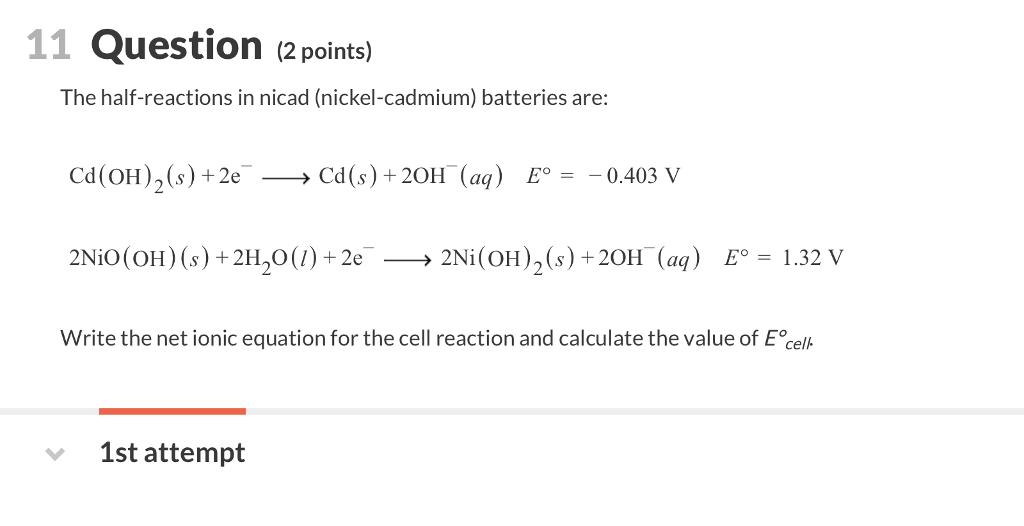
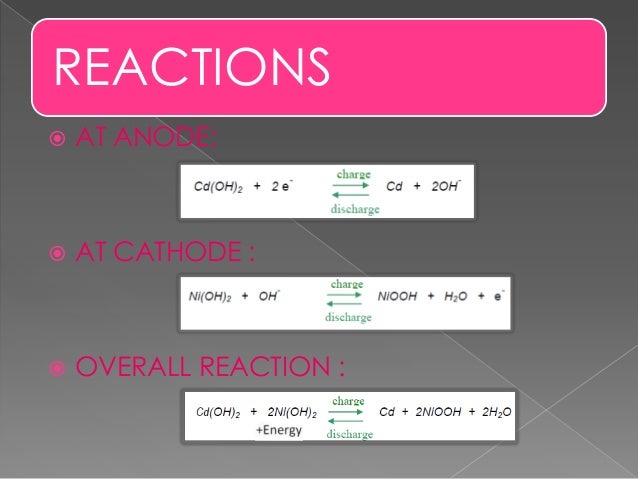
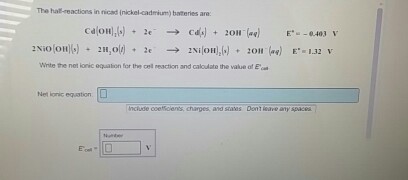
In eqns 4 6 the cell reactions during charging and discharging are presented.
However the nickel cadmium batteries are more expensive and their capacity in terms of watt hours per kilogram is less than that of the nickel zinc rechargeable batteries.
However the robust nicd batteries are very durable reliable easy to use and economical.
The abbreviation ni cd is derived from the chemical symbols of nickel ni and cadmium cd.
2015 ap chemistry free response 1d.
Nickel cadmium nicd is one of the most established amongst the various commercially available rechargeable battery systems.
Nernst equation redox reactions and electrochemistry.
They operate over a wide temperature range give approximately 2000 cycles and can be charged in less than 1 h.
The energy density of nicd batteries are lower than the newer battery systems such as nickel metal hydride and lithium ion.